Antimicrobial resistance and antibiotic use – where are we?
In Europe the use of growth promoters was banned from 2006 and now in the US, there is a bill likely to be presented to the House of Representatives, effectively to do the same thing. However, in Europe there is a further move to ban the use of antibiotics for prevention of infection, either at a low dose, given continuously or at a high dose, metaphylactically, to control infections – are we going a step too far, misguidedly?
I remember in the eighties, an antibiotic tiamulin was approved in the US for swine at 11ppm in feed for growth promotion (now no longer), 38.5 ppm for swine dysentery prevention and 220 ppm for dysentery treatment. This highlights the potential confusion and obfuscation that goes on nicely between both sides of the antibiotic resistance argument and offers a good example of what is actually going on in the pig gut.
Low levels
Antibiotic growth promoters at low levels improve the growth rate of the pig and its feed conversion efficiency, so this is positive for the producer, the nutritionist and the feed industry. Tiamulin at 11 ppm gave about 70% of the improved growth response in healthy (non-dysentery infected pigs), by bacterial inhibition of susceptible organisms in the pig’s intestine. As the dose was increased, an increase in performance could be seen up to about 30-40 ppm level and then no more, again in healthy pigs.
When pigs are infected with Brachyspira hyodysenteriae the cause of swine dysentery, 30-40 ppm was required to inhibit the growth of the bacterium and prevent the disease developing. There may be a few organisms left in the gut or picked up from environmental contamination but usually levels are well below 100-1000 (102 – 103)/ gramme of faeces i.e. below detectable levels by culture and PCR.
Disease prevention
Therefore, disease prevention is a specific claim and has a useful role in maintaining the health and welfare of the animal from a veterinary perspective (see Figure 1). When a pig has clinical dysentery with bloody diarrhoea, there are millions (>106) of bacteria/ gramme of faeces growing in the large intestine. To treat the infection high doses of antibiotic are required to kill the bacteria and reduce the level of infection, until ideally, there are no bacteria left.
However, this is not always completely possible at a farm level and depends on the severity of the condition and the damage to the gut (the chronic case) and the appetite of the pig and the subsequent intake of the drug. The susceptibility or resistance of the organism and numbers of bacteria present are also important in relation to the concentration of the antibiotic required.
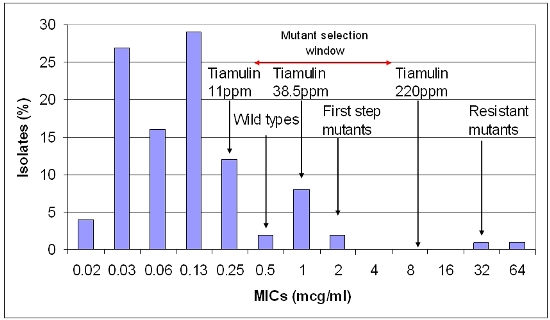
Figure 1. Relationship of drug concentration of tiamulin and B. hyodysenteriae susceptibility pattern (minimum inhibitory concentrations – MICs).
Susceptibility patterns
When we look at susceptibility patterns the pattern to the left (<0.5 µg/ml) are the so called 'wild types' which are the normal pattern of susceptible bacteria. The next wave (0.5-2 µg/ml) is the first step mutants that have developed a tolerance or some resistance to the antibiotic. These mutants may still be destroyed by high levels of the antibiotic, which has a concentration of 8 µg/g in the large gut contents when given at 220 ppm. The bacteria with MICs of 16 and 32 µg/ml are second stage mutants, which are no longer treatable with this antibiotic and are truly resistant mutants.
The zone from 0.5-8 µg/ml is the ‘Mutant Selection Window’ (MSW) and the concentration of the antibiotic, which will stop the mutation from first step mutants to resistant second step mutants, is the ‘Mutant Prevention Concentration’ (MPC). When tiamulin is used at 220 ppm it will kill not only the wild types but also the first stage mutants. The chances of a double mutation occurring at one time to produce truly resistant mutants are very low.
Risk of mutant formation
The chances of mutants to be created are increased when there are large numbers of organisms present, usually greater than 106 and therefore the risk of mutant formation and selection by the use of an antibiotic is primarily in the mutant selection window and not at low levels (growth promoter levels) below the window, where there is little selection pressure on the bacteria to develop and enrich surviving mutants. The risk of resistance development from bacterial mutation due to antibiotic use is summarised in Table 1.
Table 1. Comparative therapeutic approach and mutant selection/ resistance development risk.
Therapeutic approach | Bacterial numbers | Mutant selection window | Mutation selection risk |
Prevention levels (low dose) | Low – <106 (usually <102-3) | In (but prolonged use) | Moderate |
Metaphylaxis (high dose) | Low – <106 (usually <102-3) | In | Low |
Above | Very Low | ||
Treatment (high dose) | High – >106 | In | High |
Above | Low | ||
Combination therapy (high dose both) | High – >106 | In | Low (2 x mutation) |
Above | Very low |











