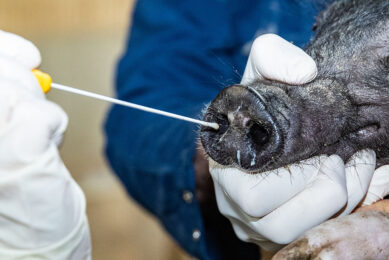
Vaccine progress swine influenza remains elusive, on-farm test is coming
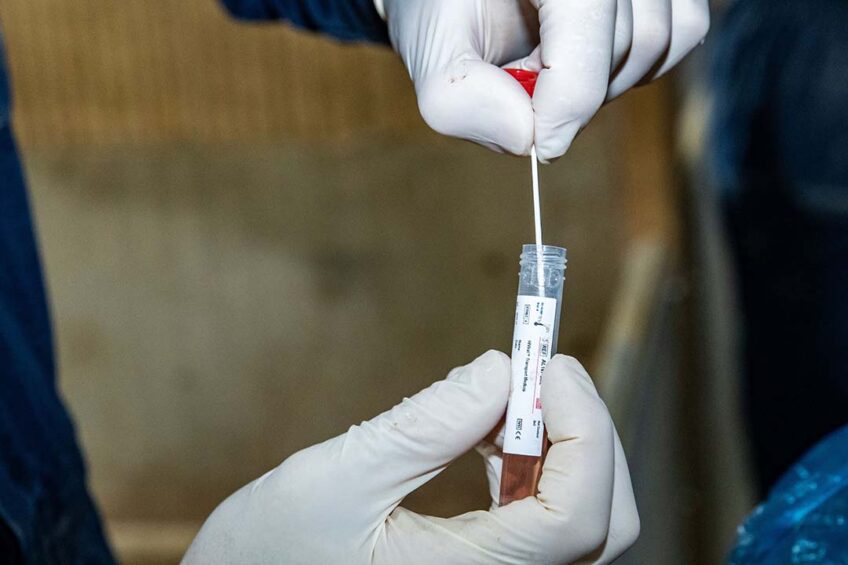
The Dutch assay developer NYtor collaborates with Alveo Technologies, a molecular sensing and diagnostics company based in California, to create a rapid, pen-side test that will detect the dominant swine flu strains that are affecting commercial herds around the world. These are H1N1, H1N2 and H3N2. Vaccine progress remains elusive.
Without an on-farm test, samples must be shipped to a lab and results may not be available for several days, helping the spread of the virus. Identification and containment of swine influenza is important, as the economic losses are severe and can be compounded by shipping infected pigs. Pigs with the disease in the growing phase can cause financial losses of up to 15%. Alveo Technologies and other partners are also close to marketing on on-farm testing kit for avian influenza as well.
Unique technology
Alveo Technologies has developed a handheld device that pairs advanced molecular assays with cloud-enabled data analytics to rapidly provide “highly-accurate and sensitive real-time detection, analysis and diagnosis of diseases, pathogens, and contaminants.” It uses Alveo’s globally patented direct-electrical sensing approach to pathogen identification with loop-mediated isothermal amplification (LAMP).
Alveo Technologies announced a partnership programme in 2023 that would enable companies in agriculture and public health to build accurate, rapid field tests to detect a wide array of pathogens, stated Shaun Holt, CEO of Alveo Technologies, in a release on 23 April. “That partnership strategy is delivering even greater results than we anticipated, with tests in development for avian influenza and now for swine flu,” he said.
Vaccine status
In a new scientific journal article, a team from University of Nebraska-Lincoln notes that vaccination remains the most effective method of controlling or preventing IAV-S (swine flu). However, progress remains elusive. They explain that “despite continued efforts in the development of a broadly-protective vaccine against IAV-S, there are extensive challenges to achieving this goal. Though, only three subtypes of IAV-S circulate in swine populations, each subtype demonstrates extensive genetic and antigenic diversity.”
The team adds that current vaccination strategies are often not updated frequently enough to address the continuously evolving nature of IAV-S. These strategies also fail to induce broadly cross-reactive responses, are susceptible to interference, may actually enhance respiratory disease, and can also be expensive to execute.
However, Dr Erika Petro-Turnquist, Dr Matthew Pekarek and Dr Eric Weaver also report that “researchers are investigating novel vaccine platforms, vector design, utilizing computationally-designed vaccine immunogens, and pursuing new-age nanovaccine technologies to increase cross-reactive responses against IAV-S.”