Getting a grip on Rosalía with PRRSv vaccination

Ever since 2020, the highly pathogenic PRRSv strain Rosalía has been present in Spain’s swine industry. While the industry has been trying to get its head around how to best face this new challenge, Rosalía has led to substantial losses for producers in Spain. Vaccination might provide one of the roads to relief.
Porcine Reproductive and Respiratory Syndrome virus (PRRSv) has been a significant cause of disease outbreaks since the late 1980s. Its effects on swine populations include reproductive failures, respiratory distress, slower growth rates and increased mortality rates. Over the years, due to the virus’s high mutation and recombination rates, more aggressive and highly pathogenic strains have emerged, causing even more severe outbreaks across different regions.
In 2020, a new highly pathogenic PRRSv strain, named Rosalía, emerged in Spain. Ever since, the productive and economic impact of this strain has been devastating. Effects include high sow mortality, persistently elevated abortion rates (up to 27% over a span of 17 weeks), and dramatically high piglet mortality rates in nurseries, with peaks ranging from 28% to 50%.
While vaccinating both sows and piglets against PRRSv has proven to reduce clinical symptoms and economic losses, the emergence of the Rosalía strain has posed a critical question: how effective are current vaccines in controlling outbreaks caused by this highly pathogenic variant?
Vaccination efficacy against Rosalía
A recent study made by Silvia Cros and colleagues of Hipra’s R&D facilities, explores the efficacy of a modified live vaccine in an experimental challenge against the Rosalía strain of PRRSv. The study involves 70 piglets (both PCR- and ELISA-negative to PRRSv), which were evenly divided into two groups.
- Group A, vaccinated intradermally (0.2 ml/dose) with a PRRSv1 modified live vaccine at 3 weeks of age; and
- Group B, which received an intradermal placebo treatment (0.2 mL of PBS) at the same age.
5 weeks after vaccination, both groups were intranasally exposed to the Rosalía strain (10e3.55 CCID50/animal). For 28 days post-challenge, the research team daily monitored and scored the clinical signs, including lethargy, anorexia, sneezing, coughing, nasal secretion, respiratory distress and skin discoloration.
The results reveal a significant 57% reduction in clinical symptoms among the vaccinated animals. These animals showed significantly milder clinical symptoms, with a clinical score of 1.73, compared to 4.04 in the control group (p value <0.001). In addition, the duration of clinical symptoms was notably shorter in the vaccinated group (see Figure 1).
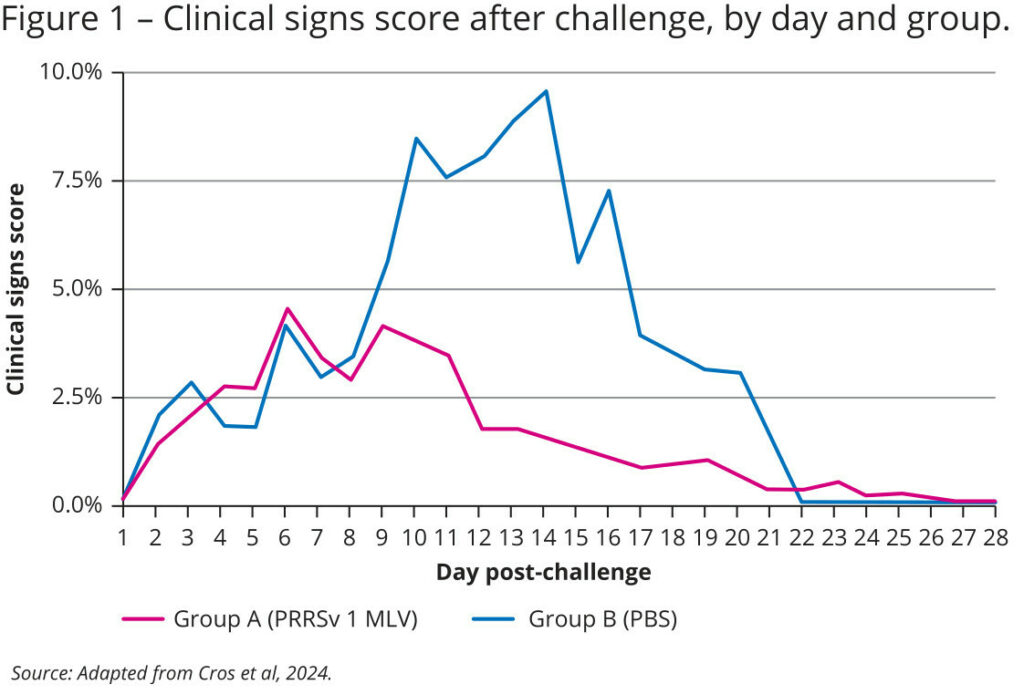
Mortality rates were also recorded and demonstrate the vaccine’s protective effect, with an 89% reduction in mortality within the vaccinated group: only 8.8% of the piglets succumbed to the virus, compared to a staggering 80% in the control group. Furthermore, all deaths in the vaccinated group occurred within the first ten days post-challenge, while the mortality in the control group was prolonged until 20 days post-challenge (see Figure 2).
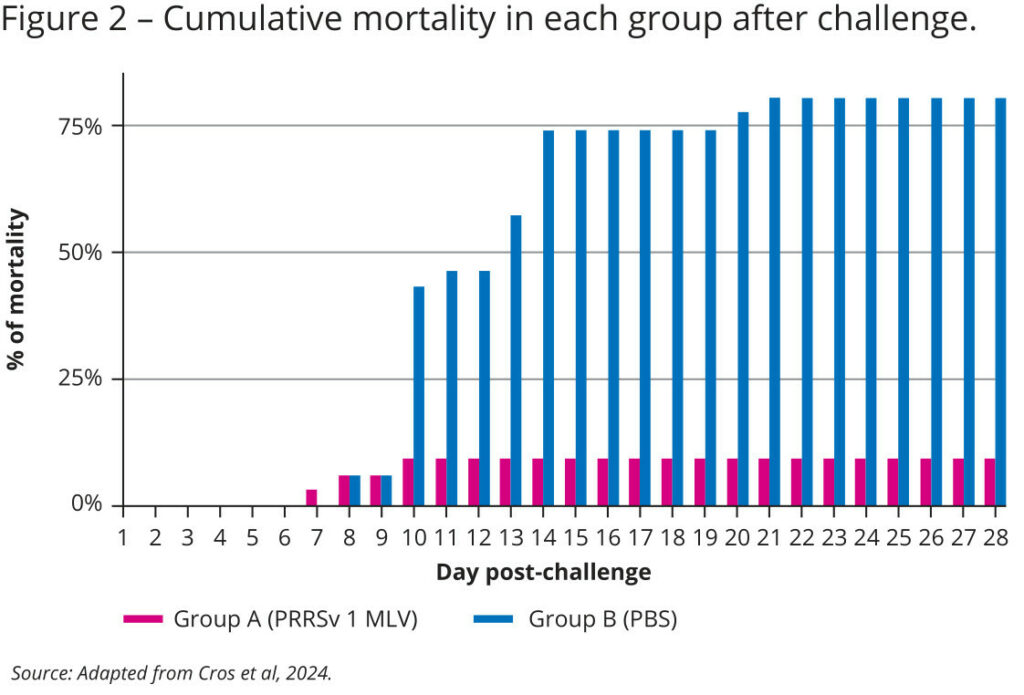
The results show a highly protective effect of vaccination, which, to our knowledge, makes this the first published study where a vaccine clearly prevents the mortality that is associated with infection by the Rosalía strain.
From the laboratory to the farm
On farms, various uncontrolled factors – such as the timing of infection, the presence of secondary infections, and environmental conditions – may influence the overall efficacy of vaccination. Therefore, to confirm the effectiveness of PRRS vaccination against the Rosalía strain, it is crucial to validate these results under real-world field conditions.
A 2024 field study published by Hipra’s technician Alba Meléndez evaluated the benefits of vaccination on a Rosalía-positive wean-to-finish farm, located in an area with a high density of swine farms. An intradermal vaccination programme was implemented at three weeks of age, using a PRRSv1 modified live vaccine, and several key performance indicators (KPIs) were monitored before and after vaccination.
Results from the trial can be viewed in Table 1. The vaccinated group showed a significant reduction in mortality (9.9% compared to 28.3% in the non-vaccinated group), along with improvements in average daily weight gain (ADWG) and feed conversion ratio (FCR). In addition, medication costs were substantially reduced (€2.18/pig). All these differences led to a €13.49 reduction in production costs for this farm.
A similar study was performed on another wean-to-finish farm, with piglet intradermal vaccination at three weeks of age, using a PRRSv1 modified-live vaccine. Besides the mortality reduction, the significant improvement in growth performance led to a €1.82/pig reduction in the production costs from weaning to slaughter (see Table 2).
A third field study conducted by the team of Meléndez reinforced these findings, showing a significant and faster reduction of the viral load in vaccinated animals on a Rosalía-positive farm, when compared with non-vaccinated animals: 34% lower at 7 weeks post-vaccination and 63% lower at 12 weeks post-vaccination. A lower viral load is linked to reduced infection pressure, fewer clinical symptoms, and improved overall performance, further emphasising the benefits of vaccination and aiding a return to pre-outbreak productivity levels.
Conclusions
Despite the aggressive nature and severe outcomes associated with the Rosalía strain, vaccination with PRRSv1 modified live vaccine has proved effective, from both health and economic perspectives.
Vaccinating piglets not only significantly reduces clinical signs and mortality but also decreases the need for antimicrobial treatments and improves production performance, with a positive return-on-investment for producers. These results are consistent with those from previous studies where vaccination showed effective against other aggressive strains, such as Lena and type 2 highly pathogenic strains.
The available data suggest that vaccinated animals cope better with the infection, exhibit shorter periods of illness and a faster return to normalcy. Vaccination remains a critical tool in managing Rosalía outbreaks, offering a practical and cost-effective solution to mitigate the impact of this highly pathogenic strain on swine production.
References available upon request.
Authors: Tiago Nunes, Lidia de Lucas, Salvador Romero, Fernando de Mergelina and Joel Miranda.





