Holistic nutritional approach for improved gut health

Measures for improving piglets’ intestinal health are of growing interest, as it is consensus that optimal gut function is the key factor for maintaining healthy and high performing animals, thereby reducing the need for post-weaning medications.
Part of the Gut Health 2022 Special
Weaning is a critical phase in young piglet’s life where numerous substantial functional changes occur in the gastrointestinal tract and the innate immunity is still insufficiently developed, making them susceptible for digestive disorders. Especially post-weaning diarrhoea as a multi-factorial problem is a pressing problem to pig farmers world-wide. Changing legislation on application of zinc oxide (ZnO) and antibiotics is further increasing the demand for alternative nutritional strategies promoting gut health.
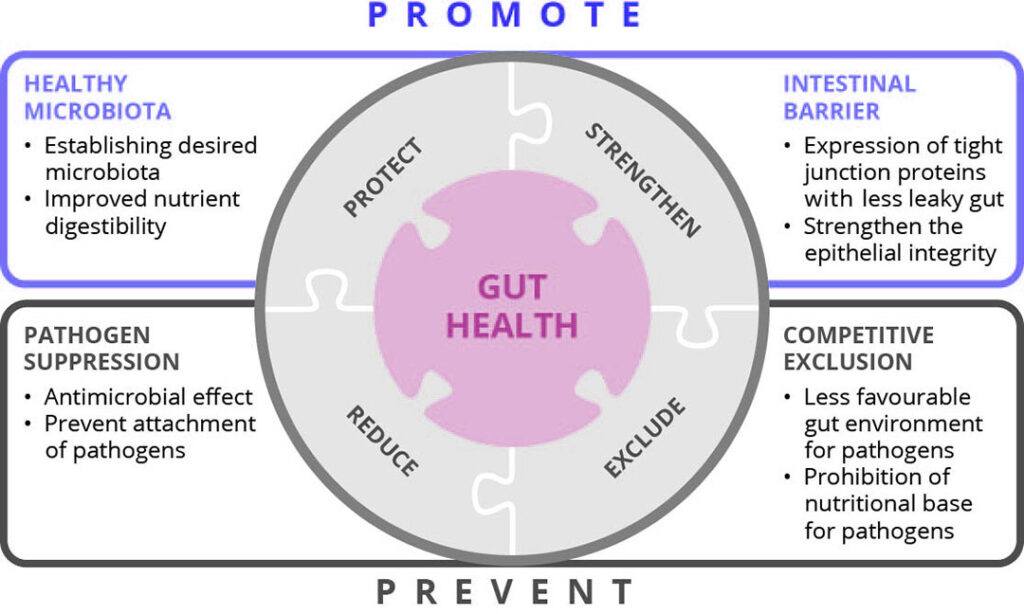
Figure 1 – Enhancing gut health with different feed additives and nutritional measurements in weaning piglets.
Gut health as the main focal point
Provita Supplements has developed a feeding strategy for weaning piglets, putting gut health as a focal point (Figure 1), that aims at strengthening the intestinal barrier function, creating a favourable environment for the establishment of a positive microbiota, and minimising the risk of pathogen attachment to the epithelium.
Key factors of this strategy are:
a) improving the proliferation of intestinal tissue due to the supply of short-chain fatty acids from desired bacterial fermentation promoted by probiotics,
b) reducing the share of undigested protein reaching the hindgut by improving dietary protein digestibility using fungal fermentation (SSF) products, and
c) reducing the pathogen prevalence in the hindgut and thus the risk of inflammation by including additives displaying a direct antimicrobial effect, such as activated ZnO (aZnO; Maxactivat/Zn).
The effect of adding aZnO to the piglet starter diet during the first 14 days post weaning was tested in several feeding trials in comparison to a conventional ZnO. As part of the trials, faeces samples of individual piglets were examined by 16s rRNA analysis to quantitatively assess the bacterial composition of the hindgut. Special attention was paid to E. coli as the main pathogen involved in post-weaning diarrhoea. Results showed a 60% decline in the average faecal E. coli count of piglets fed aZnO (1.4×109 cfu/g faeces) compared to those fed conventional ZnO (3.6×109 cfu/g faeces). Furthermore, DNA of E. coli displaying the fimbriae F4 was only detected in faeces samples of animals fed conventional ZnO, whereas this pathotype was not detected in the samples of the group treated with aZnO.
Intestinal barrier preserved
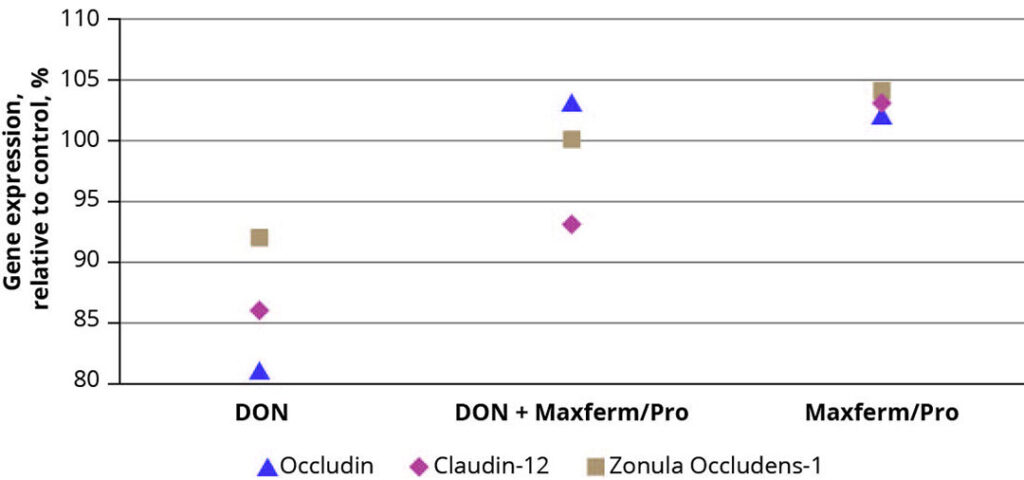
Besides the reduction of pathogens, the maintenance of the intestinal barrier against their invasion is of equal importance. This barrier is preserved by the formation of protein complexes between epithelial cells, so called tight junctions, whose expression can be impaired due to oxidative stress and toxic substances, resulting in an increased permeability of the intestinal epithelium. In a study, using a porcine IPEC-J2 cell culture, an additional effect of the protein digestibility enhancing SSF-product (Maxferm/Pro) on the expression of the tight-junction proteins occludin, claudin-12 and zonula occludens-1 was observed. The addition of deoxynivalenol (DON) to the cell culture had a detrimental effect on the expression of these tight junction proteins, leading to an 8 – 19% decrease compared to the untreated control (Figure 2). By simultaneous addition of the SSF-product, the negative effect of DON could be more than compensated, resulting in an upregulation of gene expression up to 5% compared to control. The same effect was observed in the absence of DON.
Figure 2 – Effect of Maxferm/Pro on the expression of tight-junction proteins in an IPEC-J2 cell culture.
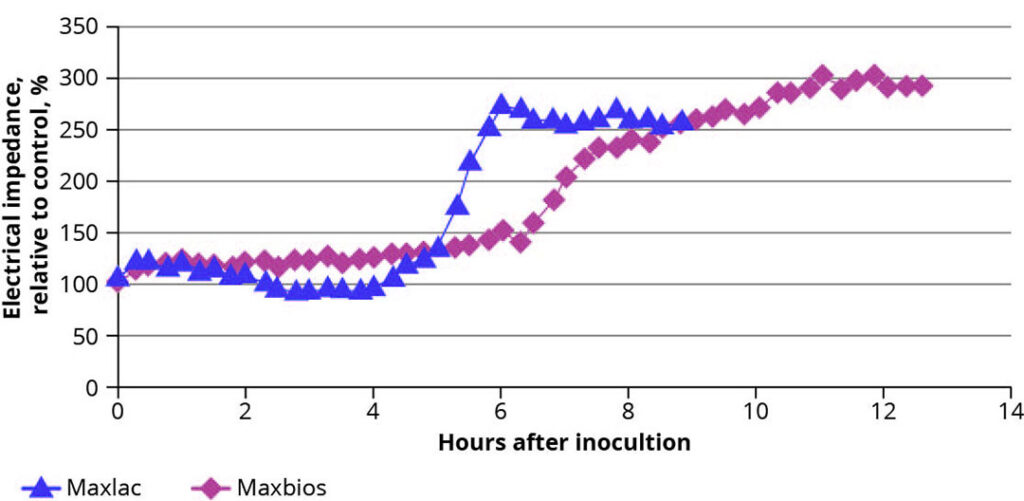
The intestinal barrier is further supported by the formation of a protective biofilm on the mucus layer, formed by lactic acid producing bacteria, which inhibits pathogens from adhering to epithelial cells. To investigate the effect of probiotics on the biofilm formation and strengthening the barrier function, another trial using an IPEC-J2 cell culture was conducted. Enterocytes were cultured on dedicated biosensors containing a high-density gold electrode array and a real time electrical impedance analysis was performed. After the formation of a stable monolayer, solutions containing either Bacillus subtilis (Maxbios) or Enterococcus faecium (Maxlac) were added and the electrical impedance was measured until a plateau was reached. Shortly after the addition of the bacterial suspensions, an increase in impedance occurred (Figure 3), suggesting an improved barrier function of the epithelial monolayer. For the strains tested, specific kinetics of the plots can be explained by the growth pattern of the respective probiotic, but a certain threshold of bacteria was needed before an effect occurred.
Figure 3 – Real-time impedance analysis in IPEC-J2 cells after addition of probiotic containing suspensions.
Antibiotic free weaning
The results of the present studies outline the efficacy of adding probiotics, SSF-products and aZnO into the diet on both an improved intestinal barrier function and a reduced pathogen prevalence in the hindgut. Aiming for an antibiotic free weaning and thus for a more sustainable pig production, it is recommended accompanying these measures by lowering the dietary protein content and including unfermentable fibre sources to the diet. This reduces the retention time of the chyme in the hindgut and removes the breeding ground for pathogens.