Better modulation of intestinal microbiota in poultry

After the ban of AGPs in Europe in 2006, use of non-medicated gut microbiota regulators as feed additives has consequently increased. In this context, a specific and patented Copper Exchanged Clay (CeC), was developed as such as an alternative
Commercial poultry production is one of the most important sources of animal protein for human consumption, not suffering from any cultural or religious restrictions and is an important economic activity in many countries, with over 60 billion birds used in the production of meat and eggs each year. Despite their strong impacts on technical and economical farms performance, little is known about microorganisms housed in the chicken gastrointestinal tract (GIT). Yet, the complex microbial community (microbiota) of the gastrointestinal tract plays a crucial role in the health of the animal and can be considered as an important metabolic “organ”. Composition of the intestinal microbiota is dynamic with spatial shifts along each GIT region in relation to environmental changes. The entire GIT of chicken is estimated to house 640 species of bacteria from 140 different genera, where about 90% of the species are yet to be described. Thus, when talking about the importance and influence of gut microflora, the number of bacterial cells in the host is 10 times the eukaryote cells number in the poultry body or that their genes number is 50 to 100 times higher than the total number of genes constituting the host.
Benefits of using AGPs
Sub-therapeutic concentrations of antibiotics, known as Antibiotic Growth Promoters (AGPs) have long been used in the food-producing industry. Their addition enhances feed efficiency, reduces mortality and improves the overall health of livestock. Their mode of action is thought to be due to a direct or indirect overall reduction or modification in bacterial numbers, as it is strongly suggested by their lack of effect on broilers. The proposed mechanisms involve a reduction of microbial nutrient utilisation, an enhancement of nutrient absorption due to a thinner mucus layer and healthy functional enterocytes, a decrease in production of unwanted bacterial metabolites such as toxins and a reduction of intestinal infections. Direct action on the host’s intestinal immune functions have also been suggested. Finally, the addition of AGP in animal feed results in a decrease of nutrient amounts needed to produce a market-size chicken and enhances birds’ growth without having negative effects on the meat quality if withdrawal delays are observed. The first evidence of AGPs performance effect dates to 1940 and has since been echoed by many studies. The development of intensive livestock farming based on the confinement of a high number of birds, therefore increases the risk of bacterial disease development and an unbalanced gut microbiota. Subsequently, AGPs have been used in routine for decades to prevent disease and improve zootechnical performances.
Their overuse has contributed to the emergence of drug resistant bacteria and to the accumulation of antibiotic residues in animal products and the environment. AGPs were banned from farming practices in 2006 in the EU, leading to an increased mortality rate, degraded techno-economical results and decreased animal welfare. This raised the need for safe and efficient alternatives that could increase nutrient availability for the animal, improve host immunity and intestinal microbiota. Thus, many feed additives have emerged in poultry nutrition such as probiotics, prebiotics, micro-elements, digestive enzymes, plants extracts or essential oils and clays.
Particularly, among clays, action of ion-exchanged clays, especially Copper Exchanged Clay on microbiota is well described and is considered a good candidates for alternative to AGPs thanks to their antimicrobial effect. Nevertheless, regarding the microbiota, most of the studies with ion-exchanged clays focus on counts of pathogenic bacteria (mainly E. coli, Clostridium and Salmonella) and do not investigate the impact of the additive on the overall microbiota balance. Consequently, in this study, we investigated the influence of a patented Copper Exchanged Clay on broilers microbiota composition compared to a negative control.
Microbiota modulation
To support poultry growth and to ensure a well-balanced and secure microflora during the whole life of the animals, a unique and patented Copper Exchanged Clay named B-Safe has been developed by Wisium. This solution is a combination of copper ions at very low level and a synthetic zeolite having anti-microbial properties and able to specifically target pathogenic bacteria and have a limited action against beneficial bacteria.
An in vivo trial was performed in partnership with the University of Rennes in France. For this research, 70 ROSS PM3 broilers were raised in provoked challenging condition with a stocking density of 45,5 birds/m² in a R&D farm from 1 to 21 days old. Half of the animals were supplemented with CeC and half of them belong to a control group (without antibiotics supplementation nor CeC). Growth performances were monitored and intestine samples from gizzard to caeca were collected at the end of the trial period to do physiological and microbiota analysis.
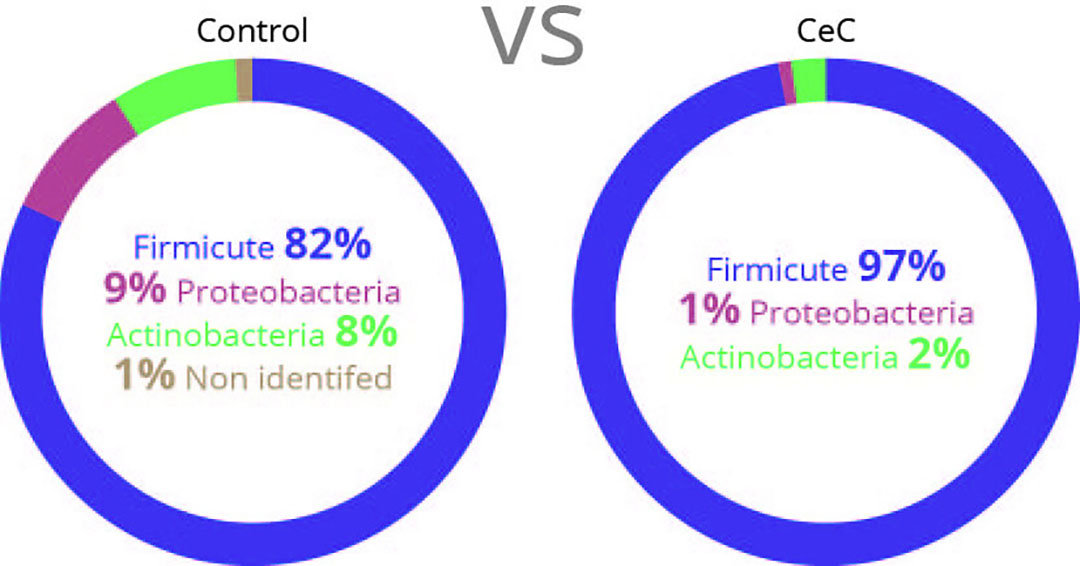
Figure 1 – Family microflora diversity in the gut of each group.
Regarding the microbiota analysis, chicken small intestinal microbiota were analysed using both VAMPS (Visualization and Analysis of Microbial Population Structures) and MG-RAST (Metagenome Rapid Annotation using Subsystem Technology) online servers. Ecology diversity was observed and taxonomic identification performed.
- CeC decreases the richness (number of bacteria species present in the gut) compared to the control. The control group counted 6 978 bacteria species whereas CeC group counted 5692 bacteria species at 21 days.
- The microflora diversity was also significantly affected by CeC: microflora profile was strongly reorientated in favour of Firmicutes accounting for 97%, and Proteobacteria (including E. coli) and Actinobacteria nearly disappeared (Figure 1).
Among Firmicute family, CeC samples were enriched in Lactobacillus compared to control group, enabled an increase in L. johnsonii, known to inhibit C.perfringens growth and colonisation by E. coli. L. reuteri, known for bacterial inhibition were not found in control group. Proteobacteria family where Shigella and Escherichia can be found were mostly reduced in CeC samples in comparison with control group (Table 1). Intestinal morphology of birds was evaluated through their length and weight. CeC significantly increased small intestine length and weight vs control group. Jejunum was the small intestine subsection the most impacted (Table 2). In term of zootechnical performances, birds supplemented with CeC had a significant higher live weight than control group animals (+1.9%). FCR was numerically improved by -0.5% by CeC supplementation demonstrating that CeC, thanks to its action on the modulation of gut microflora, allows a better nutrients absorption and so optimises animals’ performance (Table 3).
Efficient solution
Many in vitro trials clearly demonstrate the benefits of CeC against pathogenic bacteria, this in vivo trial confirmed that CeC clearly influences gut morphology and microbiota of broiler chickens by favouring the development of the commensal microflora as Lactobacillus and by controlling the development of the pathogenic microflora as E.coli. Consequently, zootechnical performances are improved.
This solution is backed up by more than 35 trials reports on broilers and demonstrates its benefits on the Average Daily Gain with an average increase by +2.9% and a reduction of the FCR by -1.8%. According to the literature, the reliability of CeC is as good as the one demonstrated with AGPs with the strong advantage that CeC does not involve bacteria resistance. Therefore, CeC is an efficient solution on poultry production, contributing to improve the performance thanks to a better modulation of the microbiota.