Medicated feed premixes – is carryover in feed really a problem?
It was reported last week that the Dutch are planning to ban the use of medicated feed premixes in 2011 from April 1st, in an attempt to reduce their overall usage of antibiotics in farm animals. It will be an interesting experiment. One of their concerns was the carryover of antibiotics from one batch of medicated feed to the next and the possibility that low levels of antimicrobial contamination may play a role in selecting for resistant strains of bacteria.
After nearly 70 years of usage of antimicrobials in man and animals, it is sometimes still amazing how much confusion there is over the cause of antimicrobial resistance and also the interrelationship between use of antimicrobials in animals and the effect it has on human resistance. It is the ‘blame game’ between human and veterinary doctors, I suspect. In a recent Dutch report (BuRO, 2010) it stated that 30-50% of human resistance came from the animal sector, which is a complete ‘myth’, and as a result, they have started to address the effect of carryover of antimicrobials in feed and to look at the effect of sub-MIC (minimum inhibitory concentrations) of antibiotics might have on Escherichia coli, as a common indicator organism.
They showed that a carryover level of 2.5% in their own model did not have an effect on antimicrobial resistance selection. This is interesting as EFSA have established a 3% carryover tolerance for anticoccidial drugs included in feed, which would seem achievable under GMP (good manufacturing practice) in most feed mills, although other researchers (RIKILT) found the carryover of antimicrobials to be in 87% of samples and anything from 0-26% of the medicated feed inclusion. However, 94% of the 140 samples were below 10% in most mills and 73% below 3%.
Using pharmacokinetic (drug concentrations in the small intestine – Burch, 2007) and pharmacodynamic (MIC – susceptibility pattern (Maran 2008, 2010) relationships, a relatively clear picture emerges. Tetracycline resistance in E. coli was used as tetracyclines account for over half of the antimicrobial usage in the Netherlands (Fidin, 2009) (see Figure 1) and chlortetracycline gut pharmacokinetics, as it is widely used in pig medicine.
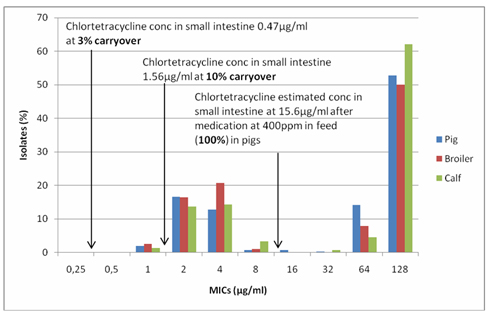
Figure 1. Comparison of gut concentrations of chlortetracycline at 100, 10 and 3% inclusion rates (theoretical levels 400, 40 and 12ppm) in feed and the tetracycline susceptibility pattern of Dutch E. coli isolates from pigs, broilers and veal calves.
At 100% inclusion there is an estimated concentration of chlortetracycline of 15.6µg/ml in the gut, at 10% 1.56 µg/ml and at 3% 0.47 µg/ml. The MICs between 1-16 µg/ml form the normal ‘wild type’ pattern, associated with no resistance, the MICs of 32 µg/ml and above are resistant mutants. The pattern is very similar for all E. coli from the 3 species of animals. These correlations are important as they demonstrate that treatment levels of drug select for resistance and not sub-MICs. In the laboratory, resistance selection is induced with concentrations of the drug around the MIC not sub MIC. The lower the concentration of the drug the less selection pressure is put on the bug to develop resistance.
When it comes to carryover of chlortetracycline in the feed, 10% would have very little effect on the susceptible E. coli and at 3% it is actually below all the MICs and would have no effect. This was confirmed in the BuRO report. This sort of pharmacokinetic/pharmacodynamic approach can also be used for other antibiotics and other bacteria.
In the main, low levels of carryover of antimicrobials in feed are likely to have little to no impact on the selection of resistance on this gut bacterium, E. coli but it is the therapeutic use that has the major impact.
Similarly, if we look at Campylobacter species (C. jejuni is mainly associated with chickens and C. coli primarily from pigs) the former being the major cause of ‘food poisoning’ in man, a similar pattern can be seen (see Figure 2).
Figure 2. Susceptibility pattern of C. jejuni and C. coli against tetracycline and comparison with chlortetracycline concentrations in the small intestine of pigs.
As Campylobacter are slightly more susceptible to tetracyclines, a 10% carryover could have a potential impact on some of the more susceptible isolates. This would need to be tested. However, a 3% carryover can be predicted to be unlikely to have any impact but this also would need to be confirmed experimentally. Antibiotics do bind to substances in the gut contents such as proteins, which can reduce their bioavailability.
Simply, if you use antibiotics therapeutically, you are likely to select for resistance. The susceptibility patterns that have been carved out over the years, whether in man or in animals are primarily a result of the therapeutic use in those particular species and are usually quite different. Doctors use as much antibiotic in man as veterinarians do in animals. There is some overlap, especially in food transmitted bacteria such as Campylobacter and Salmonella, with estimated case rates of about 0.6 and 0.1% of the human population/annum, respectively. This is relatively minor in comparison with what the doctors can cause on their own – how many of you have had antibiotics this winter for colds and influenza? MRSA (methicillin resistant Staphylococcus aureus) in dogs was initially transmitted from man to their pets – a reverse transmission. In the UK, a House of Lords (1998) review of ‘Resistance to Antibiotics’ was carried out and the medical doctors were surprised at how little resistance actually arose from animal antibiotic use (Wise – personal communication) – it is more likely 3-5% not 30-50%.











