CVMP positive towards Ingelvac CircoFLEX
The Committee for Medicinal Products for Veterinary Use (CVMP) adopted a positive opinion at the end of last year on recommending to grant marketing authorisation for the veterinary medicinal product Ingelvac CircoFLEX.
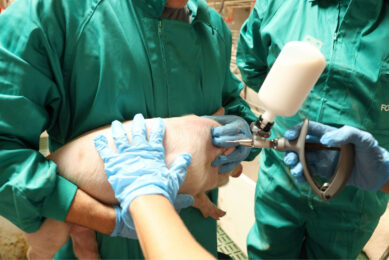
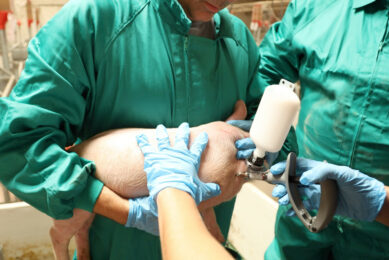
Ingelvac CircoFLEX is a product of Boehringer Ingelheim Vetmedica GmbH and is intended for the active immunisation of pigs, over the age of 2 weeks, against porcine circovirus type 2 (PCV2).
The active substance of Ingelvac CircoFLEX – inactivated porcine circovirus type 2 ORF2 protein – stimulates an active immune response to porcine circovirus type 2. Protection can begin as early as two weeks after vaccination and can last at least 17 weeks.
Benefits of the product lie in reduced mortality and in clinical signs such as weight loss and lesions in lymphoid tissues. In addition, vaccination has been shown to reduce PCV2 nasal shedding, viral load in blood and lymphoid tissues and the duration of viraemia.
Detailed conditions for the use of this product will be described in the Summary of Product Characteristics (SPC) which will be published in the European Public Assessment Report (EPAR) and will be available in all official European Union languages after the marketing authorisation has been granted by the European Commission.
Related websites:
• CVMP