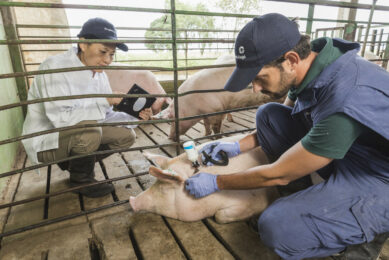
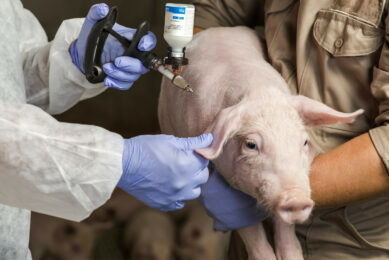
One-dose vaccination against M. hyo and PCV2

Mycoplasma hyopneumoniae (M.hyo) and Porcine Circovirus Type 2 (PCV2) are the two most prevalent pathogens that are encountered in the pig industry. Vaccination against M. hyo is already standard practice, and the same will be the case for PCV2.
By Alex Eggen, Ulrike Schmidt, Maurice Raes and Maarten Witvliet. Intervet/Schering-Plough Inter-national, Boxmeer, the Netherlands
As vaccines against these two pathogens are preferably given at a young age, it would be advantageous if vaccinations against these two pathogens are combined. However, no interference between vaccinations is acceptable as that could lead to poor vaccine efficacy. In a previous study, it has been shown that the Intervet/Schering-Plough Animal Health vaccines Porcilis M. Hyo and Porcilis PCV can be given concurrently without any negative consequences for the efficacy. Also, the efficacy and safety of simultaneous use (mixing) of both vaccines has been evaluated in the field without evidence of interference or apparent safety problems. Here, we report a series of studies on possible interference between vaccination with Porcilis PCV and M+PAC, and the efficacy of especially prepared ready-to-use M. hyo-PCV2 combination vaccines.
©
Materials and methods
Groups of eight conventional piglets, with maternally-derived antibodies against both M. hyo and PCV2, were vaccinated at weaning (three to four weeks of age) with M+PAC (and Porcilis PCV), or with ready-to-use M. hyo-PCV2 combination vaccines. At seven weeks of age, the pigs were infected with an M. hyo field isolate. Challenge infection was performed with a culture of strain 98 (obtained from Dr N. Friis, Danish National Veterinary Laboratory) by intratracheal inoculation on two consecutive days, followed by necropsy to evaluate M.hyo-induced lung lesions (LLS) three weeks later.
©
Serum samples taken at the time of vaccination and at ten weeks of age were analysed for antibody titers against PCV2 in a specific Elisa. The vaccines were based on M+PAC (Emunade adjuvant) and Porcilis PCV (XSolve adjuvant). Both adjuvants are emulsions of light mineral oil in water, but Emunade also contains alhydrogel, and d,l-α-tocopheryl acetate is part of the XSolve adjuvant. Combination vaccines were formulated with the same antigens that are used in the single vaccines. For M. hyo either the antigen that is used in M+PAC (Mh1) or in Porcilis M Hyo (Mh2) was used.
©
Results and discussion
As shown in Table 1, all vaccines tested provided similar levels of protection against M. hyo (53% – 61% reduction in lung lesions) after challenge at seven weeks of age. There were no significant differences between the vaccinated groups. Whereas the mean antibody titers against PCV2 had declined in the M+PAC only and control groups from 7.4 log2 at the time of vaccination to 3.8 and 3.7 at ten weeks of age, all animals that had been vaccinated against PCV2 showed a titer increase at ten weeks. Piglets that have an antibody titer of 11.9±2.1 at ten weeks of age after vaccination with Porcilis PCV at three weeks are protected against PCV2 challenge at the end of the fattening period. In the present study, all groups were at or above that antibody level. It can therefore be concluded that there is no immunological interference between the M. hyo and PCV2 antigens of M+PAC, Porcilis M Hyo and Porcilis PCV, independent of whether they are given concurrently as separate single vaccines or combined in a ready-to-use vaccine with either Emunade or XSolve as the adjuvant.
©
Further proof for lack of interference comes from a recent field study in which M+PAC and Porcilis PCV were mixed just before administration. There was no significant difference between the anti-PCV2 antibody titers in groups of pigs that were vaccinated with the mixed products or that received the two vaccines by separate injections.
©
References available on request.
©