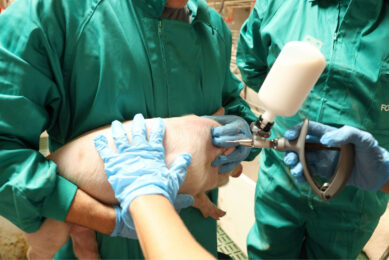
Injectable iron products for pigs often contain impurities
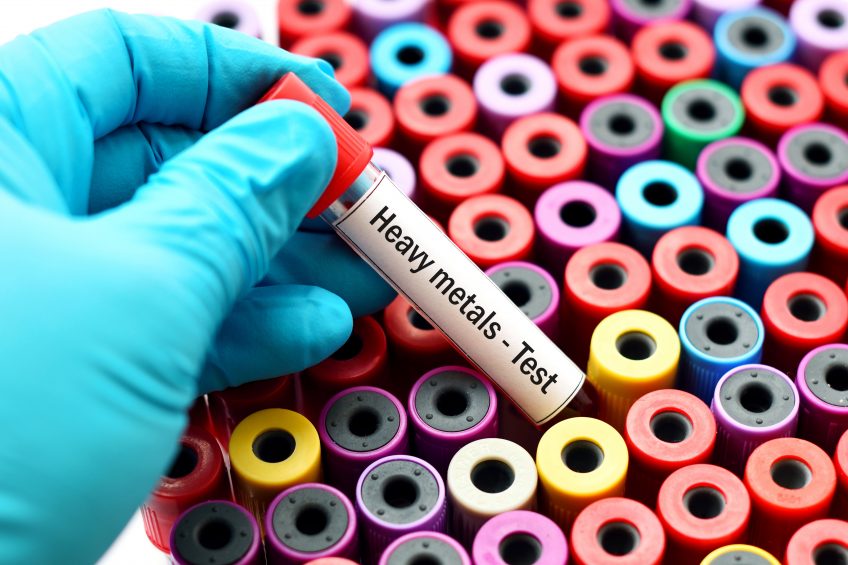
Injectable iron products for swine often contain levels of arsenic, chromium and lead which are at concentrations above the exposure limits for humans.
That was communicated by researchers of Iowa State University in cooperation with Danish injectable iron company Pharmacosmos, in a publication that appears in the May/June edition of the peer-reviewed Journal of Swine Health and Production. This publication is published by the American Association of Swine Veterinarians.
Nearly an industry standard in swine production
The authors wrote in their article that the use of injectable iron for the prevention of iron deficiency anaemia is ‘nearly an industry standard in swine production throughout the world’.
They wrote, “Since initial reports in the mid-20th century detailed a piglet’s need for supplemental iron, 200-mg doses of injectable iron have routinely been given to every pig as per product label directions.” They added that often, an additional 200 mg dose of iron prior to weaning has been shown to provide improved post-weaning growth performance.
The aim of the research was to make sure that iron products are safe, efficacious and consistent – and to evaluate parenteral veterinary iron products.
16 iron products from 8 countries
The researchers wrote, “In total, 16 iron products from 8 countries, each approved for the treatment of iron deficiency anaemia in swine, were evaluated by the Toxicology and Nutrition Laboratory at the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL). 15 of the 16 samples were also evaluated for the same analytes at an independent laboratory.”

Read more about pig health in the Pig Health tool
Under the conditions of this study, most of the 16 injectable iron products tested contain levels of arsenic, chromium or lead exceeding the human parenteral daily exposure (PDE) levels for each respective impurity.
Undetectable concentrations of heavy metals
Only 1 product – Uniferon by Pharmacosmos, the company that delivered a co-author for the article – possessed concentrations of all 3 elements of concern that were undetectable or below the parenteral daily exposure limit for humans for each heavy metal, respectively.
The article in Journal of Swine Health and Production was written by Scott L. Radke and Steve M. Enley, Iowa State University, Ames, IA, United States; and Chris W. Olsen, Pharmacosmos.