Why in-feed mycotoxin contamination analysis is recommended?

When it comes to Toxin-inactivation strategy, nothing is more important than the accuracy of mycotoxin analysis method. In-feed mycotoxin testing, had been a common analysis method as part of many toxin-inactivation strategies with the support of registered and effective product to in-activate the measured (in-feed) mycotoxins (e.g., MiaBond 360). By in-feed mycotoxin testing the complex mycotoxin metabolism after feed intake by livestock will be skipped resulting higher accuracy of the exact contamination.
It is important to review the complex metabolism of mycotoxins after consumption.
Among all other important duties of Gut Intestinal Tract (GIT), its role for in-feed mycotoxins absorption is a very important point needing attention. The absorption rate and therefore bioavailability of different mycotoxins after consumption by animal varies due to many factors such as mycotoxin characteristic and mode of actions.
Mycotoxin absorption
Majority of mycotoxins are absorbed in the upper part of the GIT and they have different speed for absorption rate. Aflatoxin (AFL) as an example, could be absorbed much faster through the GIT whereas Fumonisin (FUM) has the very limited absorption in the GIT. Trichothecenes (e.g, DON) and Ochratoxin (OTA) have lower absorption in comparison with AFL.
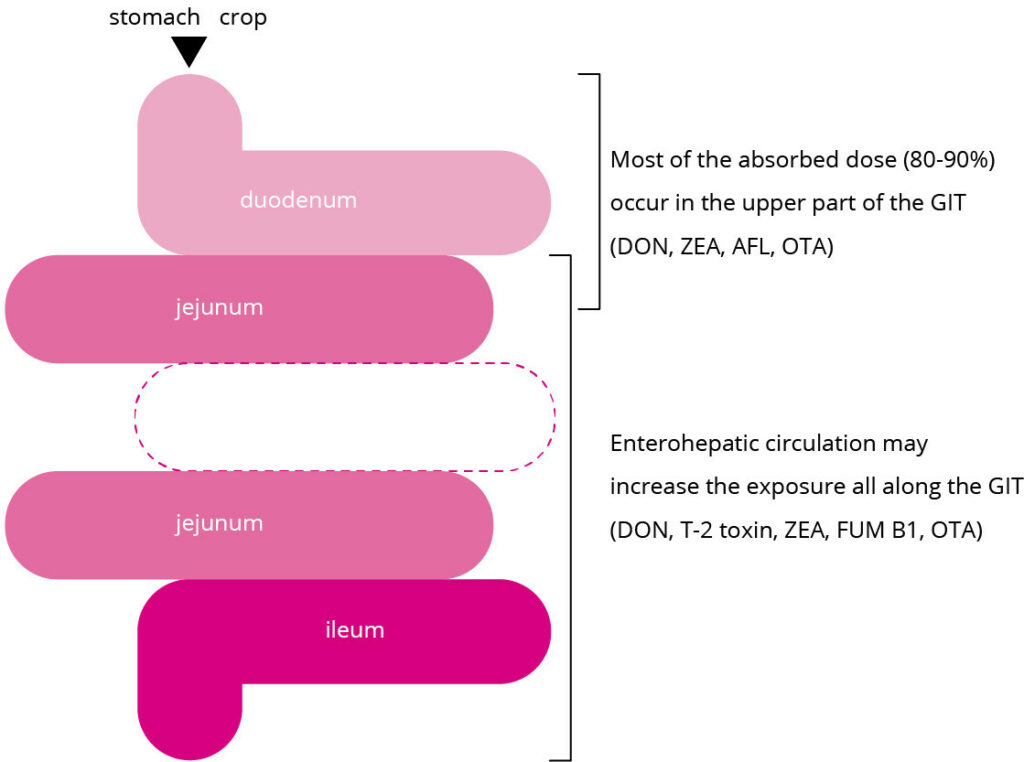
DON as an example, could be absorbed firstly via blood stream and then re-absorbed in the intestinal lumen.
Mycotoxin’s metabolism could be affected by the entero-hepatic circulation which then would increase the exposure all along the GIT. This means that mycotoxin (e.g., FUM) could be re-absorbed and make more damage to the GIT although the absorption rate of it through GIT is limited.
This very complex process of mycotoxins absorption/ metabolism needs to be considered when deciding on different method than “in-feed” mycotoxin contamination testing. One method which is not based on testing the contaminated feed directly is biomarker.
Biomarker methods
There are two different methods for biomarkers: Exposure-based and Mechanism-based where mycotoxins can be measured in fluid/ tissue and by specific biological response respectively.
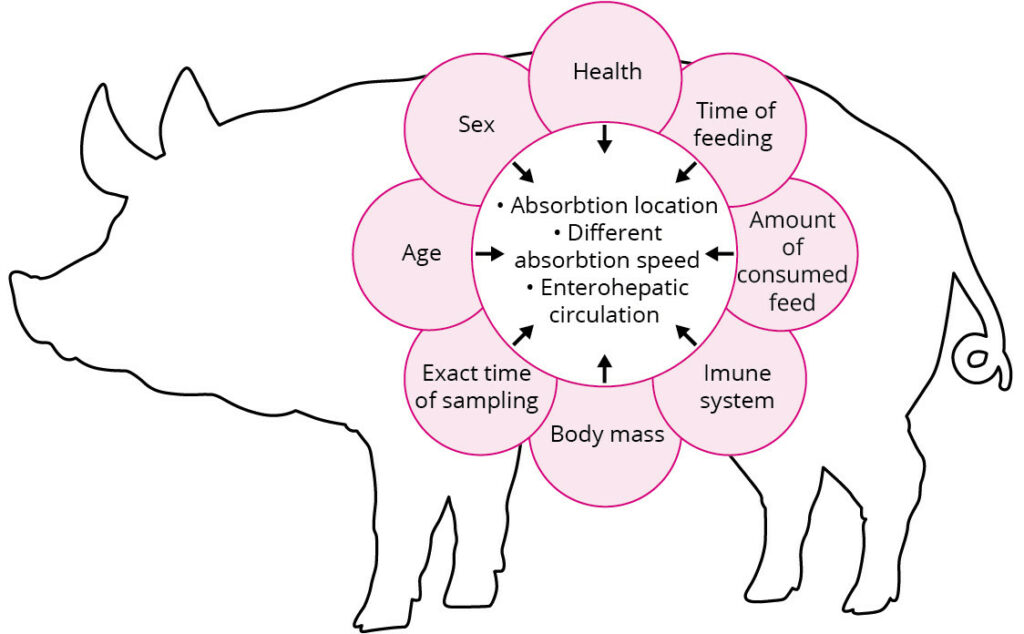
Worth to mention that both methods are dependant to the mycotoxin absorption/ metabolism in the body of the animal.
This absorption/ metabolism of mycotoxins is complex and highly dependent to many factors such as; the age, sex, health, the amount of consumed feed, body mass and immune system of the targeted animal.
Meaning that the analysed number of mycotoxins by biomarkers detection could be not precise and is quite dependant to many different factors (explained above).
Also, some mycotoxins (e.g. DON) could be present in feed but due to several reasons they could not detectable in the blood which is showing no strong correlation in between mycotoxin in feed and in the blood.
Exact time of sampling
In addition, due to the fact that different mycotoxins have different speed of absorption and that the animal conditions (mentioned above) have strong effect on mycotoxin exposure, the result of one fluid/ tissue sampling does not represent the precise number of each detected mycotoxin. As an example, after consuming contaminated feed with DON, the concentration of this mycotoxin rises relatively quick to a maximum level in the blood and then drops steadily. Showing the importance of the exact time of sampling for mycotoxin detection in biomarker assay.
Furthermore, there is no strong correlation in between mycotoxin amount in blood and the clinical sign after mycotoxin contamination.
Last but not the least, there are yet so many missing non-regulated mycotoxins to be evaluated by biomarkers in comparison with in-feed mycotoxin analysis tests (HPLC method).
In-feed mycotoxin analysis
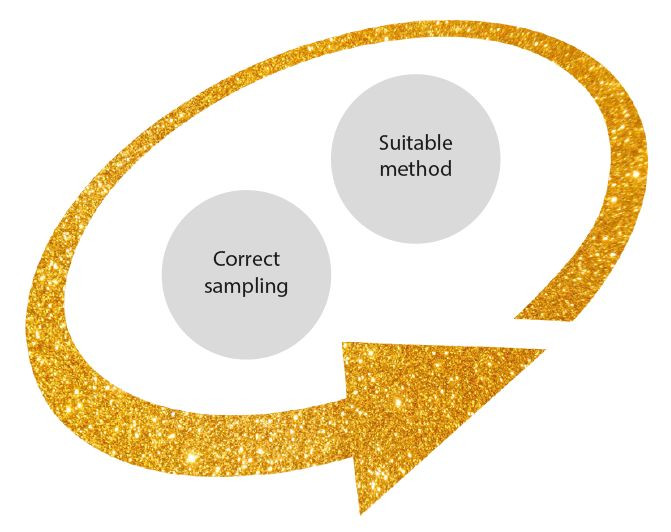
In conclusion, we would like to emphasise that in biomarker analysis, the correlation in between the exact amount/ time of mycotoxin exposure, sampling (blood/tissue) and animal dependent factors can severely affect the accuracy of the obtained results. We recommend to detect mycotoxins by a method which is not dependant to the mycotoxin absorption/ metabolism in the body of the animal. Therefore, In-feed mycotoxin analysis testing is just dependant to two different factors included correct sampling and selecting the correct analysis method.
For more information please directly contact: Toxininactivation@miavit.com
References are available on request.



