The ‘glowing future’ of FMD research
Foot-and-mouth disease occurs in all countries around the world, having a health and economic impact on pig and other livestock production. A novel method of determining the replication characteristics of the virus is hoping to minimise the future impact of this potentially devastating disease.
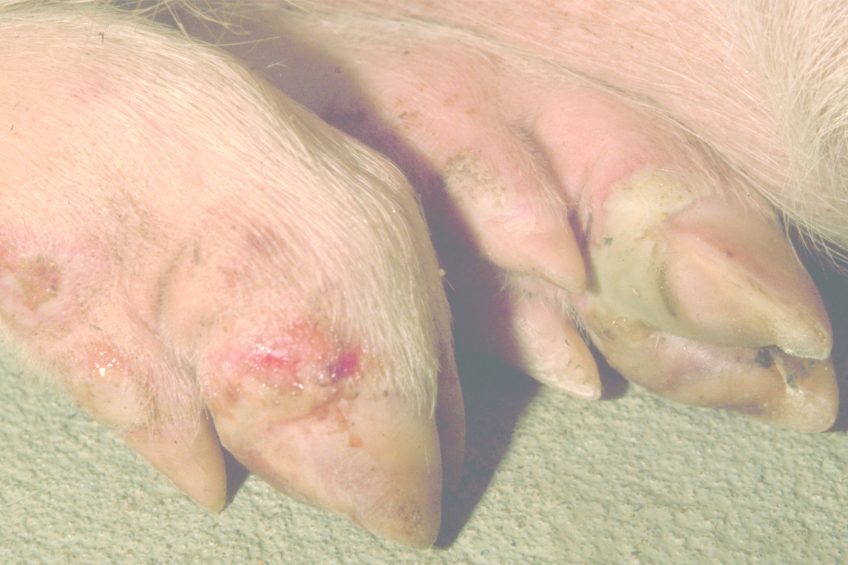
Foot-and-mouth disease (FMD) is a severe, highly infectious disease. FMD is most well known as a disease of cattle, but also affects a plethora of domestic livestock, most notably sheep and pigs in the UK, as well as wild cloven-hoofed animals such as wildebeest. FMD in adult animals is not normally fatal. However, an outbreak in a population of livestock can have morbidity rates reaching 100%, causing fevers, weight loss, abortions and lameness. The disease also has a notably higher mortality rate in infant animals. Unfortunately, FMD usually results in the slaughter of all infected animals as well as those that have been in close contact with infected animals. As a result, outbreaks in disease-free countries such as the UK can lead to a significant economic impact.
FMD has been reported in all countries throughout the world with the exception of New Zealand. Frequent outbreaks have occurred recently within a number of countries worldwide including South Korea and Russia. Outbreaks in lower income countries often become a significant issue, partly due to the reliance on small holdings of livestock and self-sufficient farming. The UK has not had an outbreak since 2007, but has a history of significant occurrences. These include the notable 2001 outbreak which saw the culling of some 6 million animals and an estimated cost of £8 billion to the UK economy.
Foot-and-mouth disease virus
FMD is caused by infection with a virus called foot-and-mouth disease virus (FMDV) which is closely related to several viruses of humans such as rhinovirus, which causes the common cold (Image 1). The disease-causing viruses are small (many millions can fit on the head of a pin), each one containing genetic material (the viral genome) encased in a protein shell or capsid. During an infection, the genetic material is released into a host cell, where viral proteins are made. This process results in taking over the normal functions of the cell and turning this into a ‘factory’ for making more virus. This process of so-called ‘replication’ is very fast for FMDV, taking only a few hours and can explain why the disease can spread so rapidly between animals.

To help prevent the spread of infection, vaccines are available in the form of inactivated viruses. However, many countries choose to forgo vaccination for several reasons. These include the cost and organisational difficulties of vaccinating animals dispersed over the countryside, as well as the near impossible task of vaccinating wild animals who may roam freely across regional and national borders. Immunity against FMDV is also complicated by the existence of seven distinct serotypes (O, A, C, SAT 1, SAT 2, SAT 3 and ASIA 1) as well as sub-types that do not offer cross protection. This makes the generation of vaccines difficult, as to be effective, they must successfully induce an immune response to multiple serotypes. There are also some inherent risks with using inactivated viral vaccines including the difficulties with achieving complete inactivation during vaccine production and the risk of accidental escape of virus from vaccine production facilities.
Virus-free models for safe research
The requirement for new technologies to combat FMD outbreaks is highlighted by the continued reporting of outbreaks across multiple countries every year. There is therefore a need to conduct basic research that leads to improved therapeutic strategies. Novel research into these much needed approaches, including both vaccine and antiviral drugs, is occurring in laboratories worldwide. However, the use of ‘live’ infectious virus in research is restricted to a handful of key high containment facilities worldwide, for safety reasons.
In recent years there has been the development of novel ways to investigate how the virus makes copies of itself in cells (i.e. the process of replication introduced previously) without the need for producing infectious virus. The University of Leeds is utilising a model that allows the effective study of replication, whilst eliminating the risks of working with infectious virus. This system removes the capsid proteins, essential for the movement of virus both between cells and between animals, and replaces them with a safe fluorescent protein, creating what is known as a ‘replicon’.
This technique is increasingly popular for the study of many infectious pathogens worldwide due to the lack of infectivity making it a safe way to study viruses. Replacing the capsid proteins with a fluorescent protein allows easier monitoring of the replication of the FMDV genetic material within cells using fluorescent microscopes (Image 2).

Working in this simple system allows researchers to investigate complicated aspects of virus life-cycles in detail; helping discover potential targets for new therapeutics. When compared to larger and more complex organisms such as animals and plants, viruses have smaller genomes which encode for only a small number of proteins. Yet viruses must use this limited set of proteins to complete their entire life-cycle. As a result, many viruses such as FMDV have evolved different mechanisms which maximise functionality from a small number of proteins. This is sometimes known as having an ‘economical genome’. Researchers at the University of Leeds recently identified that a tiny protein produced by FMDV during infection has a major role in the replication of the virus. Studying this protein – called 3B3 – the researchers (together with collaborators) identified that 3B3 has 2 separate but essential functions during replication. In doing so a new level of genetic economy used by these viruses was revealed which had not been previously reported.
Discovering these, previously unknown, mechanisms used by the virus during its life-cycle helps increase the understanding of how FMDV replicates and causes disease, providing key new information for designing new safe vaccines systems or new anti-viral agents.











